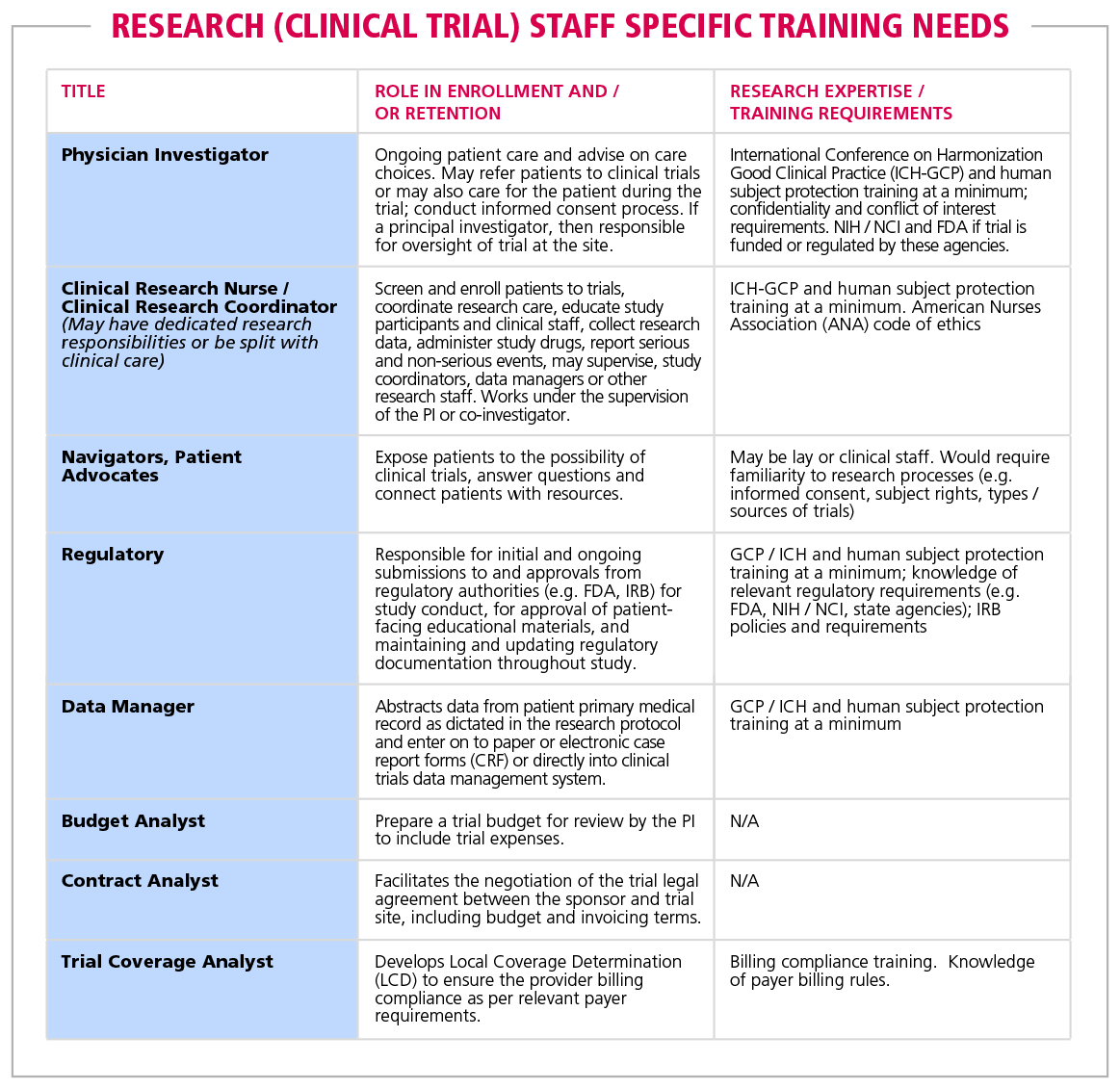
Table 2. Research (clinical trial) staff specific training needs | American Cancer Society Cancer Action Network

Table 2 from Creating Clinical Trial Summary Tables Containing P-Values : A Practical Approach Using Standard SAS ® Macros | Semantic Scholar

Table 1 from Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review | Semantic Scholar
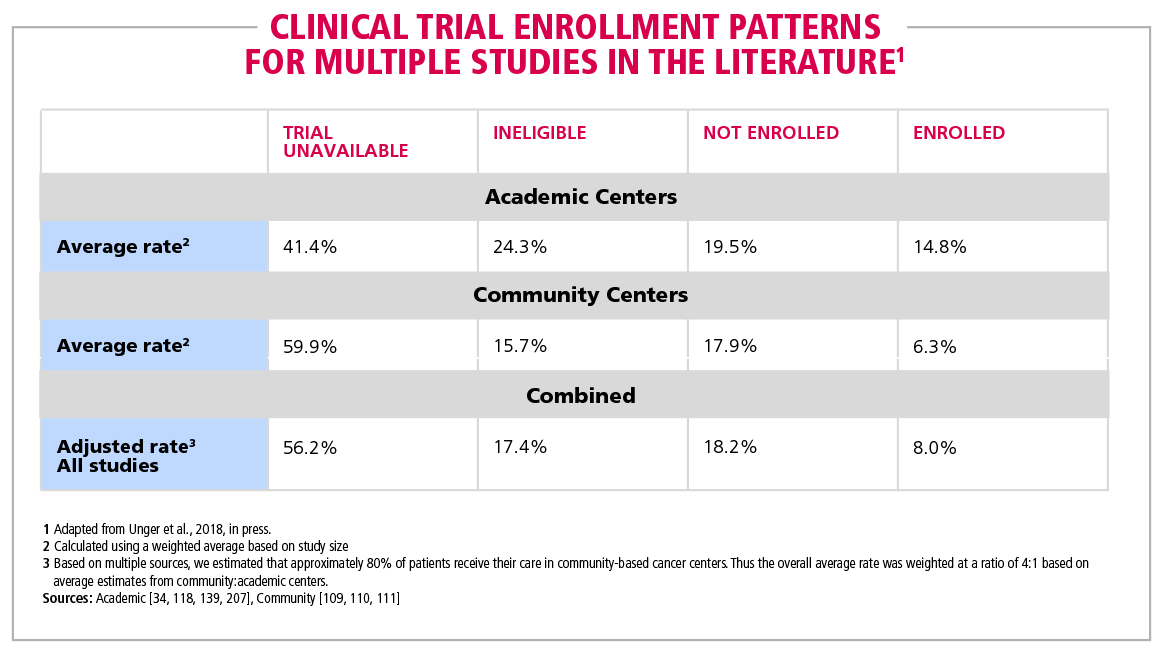
Table 1. Clinical trial enrollment patterns for multiple studies in the literature | American Cancer Society Cancer Action Network